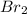Answer: At equilibrium , there are 0.274 moles of
Step-by-step explanation:
Moles of
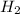 = 0.682 mole
= 0.682 mole
Moles of
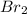 = 0.440 mole
= 0.440 mole
Volume of solution = 2.00 L
Initial concentration of
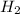 =
=
Initial concentration of
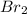 =
=
Equilibrium concentration of
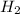 =
=
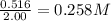
The given balanced equilibrium reaction is,
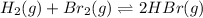
Initial conc. 0.341 M 0.220 M 0 M
At eqm. conc. (0.341-x) M (0.220-x) M (2x) M
Given : (0.341-x) M = 0.258 M
x= 0.083 M
Thus equilibrium concentartion of
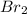 = (0.220-0.083) M = 0.137 M
= (0.220-0.083) M = 0.137 M
Thus moles of
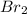 at equilibrium =
at equilibrium =
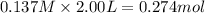
At equilibrium , there are 0.274 moles of