Answer:
85.952 ml
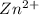 needed to titrate the excess complexing reagent .
needed to titrate the excess complexing reagent .
Step-by-step explanation:
Lets calculate
After addition of 80 ml of EDTA the solution becomes = 20 + 70 = 90 ml
As the number of moles of
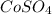 =
=
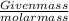
=
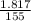
=0.01172
Molarity =
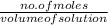
=
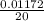
=0.000586 moles
Excess of EDTA = concentration of EDTA - concentration of CoSO4
= 0.009005 - 0.000586
= 0.008419 M
As M1V1 ( Excess of EDTA ) = M2V2
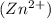
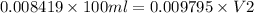
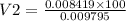
V2 =85.952 ml
Therefore , 85.952 ml
 needed to titrate the excess complexing reagent .
needed to titrate the excess complexing reagent .