Answer: pH of buffer solution is 8.1
Step-by-step explanation:
The formula for the Henderson–Hasselbalch equation is:
![pH=pK_a+\log([A^-])/([HA])](https://img.qammunity.org/2022/formulas/chemistry/college/mrp6tg63mhnvgxx26y8zuu5384c6s7jeq3.png)
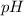 is the concentration of
is the concentration of
![[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/869xv4va2pom353pcqwbgfhb9dbdu5d2fd.png)
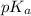 is the acid dissociation constant,
is the acid dissociation constant,
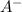 and
and
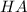 are concentrations of the conjugate base and starting acid.
are concentrations of the conjugate base and starting acid.
Putting in the values we get:
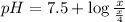
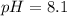
Thus pH of buffer solution is 8.1