Answer:
25 minutes
Step-by-step explanation:
V = Volume =
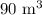
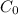 = Radon concentration under steady state = 1.5 Bq/L
= Radon concentration under steady state = 1.5 Bq/L
k = Radon decay rate =
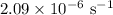
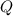 = Venting rate =
= Venting rate =
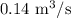
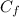 = Final concentration of radon = 0.15 Bq/L
= Final concentration of radon = 0.15 Bq/L
Theoretical detention time is given by
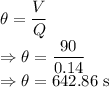
We have the relation
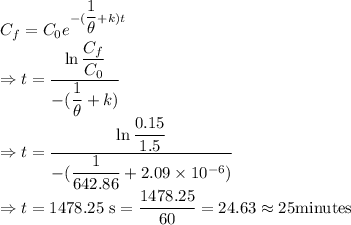
The time taken to reach the acceptable level of concentration is 25 minutes.