Answer:
E
Step-by-step explanation:
We want to determine the amount of methanol (CH₃OH) needed to prepare 175 mL of a 1.00 M CH₃OH solution.
First, determine the amount of moles of CH₃OH needed. Recalled that molarity is simply moles per liter volume (mol / L):
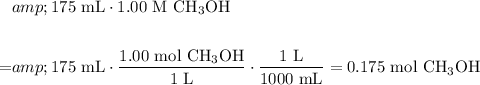
Convert from moles of CH₃OH to grams:
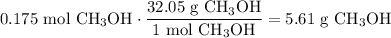
In conclusion, the answer is E.