Answer: The percentage composition of carbon and hydrogen in the compound are 64.4 % and 10.9 % respectively
Step-by-step explanation:
Mass of
 = 8.03 g
= 8.03 g
Mass of
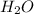 = 3.34 g
= 3.34 g
Molar mass of carbon dioxide = 44 g/mol
Molar mass of water = 18 g/mol
For calculating the mass of carbon:
In 44g of carbon dioxide, 12 g of carbon is contained.
So, in 8.03 g of carbon dioxide, =
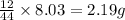 of carbon will be contained.
of carbon will be contained.
For calculating the mass of hydrogen:
In 18g of water, 2 g of hydrogen is contained.
So, in 3.34 g of water, =
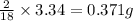 of hydrogen will be contained.
of hydrogen will be contained.
Mass of oxygen in the compound = (3.4) - (2.19+0.371) = 0.839 g
percent composition of C =
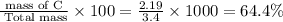
percent composition of H =
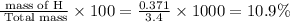
Thus the percentage composition of carbon and hydrogen in the compound are 64.4 % and 10.9 % respectively