Answer:
Relative molecular mass of copper (II) sulphate crystals is 159.611 g/mol
Step-by-step explanation:
The chemical formula of copper (II) sulphate crystals is CuSO4
Molar Mass of Cu or copper - 63.546 g/mol.
Molar Mass of S or sulfur - 32.065 g/mol.
Molar Mass of O or oxygen - 16 g/mol. Hence, Mass of four oxygen molecule would be equal to 64 g/mol.
Total mass of copper (II) sulphate is
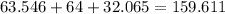 g/mol
g/mol