Answer:
![[H^+]=1.12x10^(-6)M](https://img.qammunity.org/2022/formulas/chemistry/college/g1cxmr8gxd0ymcmo4w5rakyydiyh9mn55p.png)
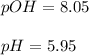
Step-by-step explanation:
Hello there!
In this case, since the question is incomplete, but given the concentration of the hydroxide ions in the solution you are given, it is possible for us to calculate pH, pOH and [H+] as signals of acidity or basicity for the analyzed solution:
![pOH=-log([OH^-])=-log(8.9x10^(-9))=8.05\\\\pH=14-pOH=14-8.05=5.95](https://img.qammunity.org/2022/formulas/chemistry/college/468qcnq64gkajvtqubyreny8fus1grbf7y.png)
Next, once the pH is calculated, we can do the same for the concentration of hydrogen ions in the solution:
![[H^+]=10^(-pH)=10^(-5.95)=1.12x10^(-6)M](https://img.qammunity.org/2022/formulas/chemistry/college/o6qxyn3e5jf0dpva6ma5j3jo8ue8bton2z.png)
Best regards!