Answer:
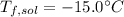
Step-by-step explanation:
Hello there!
In this case, for these problem about the colligative property of freezing point depression, it is possible set up the following equation:
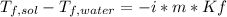
Whereas the van't Hoff's factor, i, is 2 since KCl is ionized in two ions (K+ and Cl-); and the molality (m) of the solution is computed by:
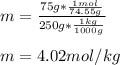
Thus, since the freezing point of water (ice) is 0°C, we obtain the following freezing point of the solution by plugging in:
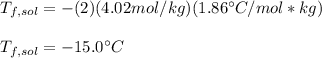
Best regards!