Answer:
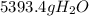
Step-by-step explanation:
Hello there!
In this case, for the described chemical reaction, we can write:
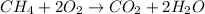
Thus, by considering the 1:2 mole ratio of methane to water, and the molar mass of the latter (18.02 g/mol), the following is useful to calculate the mass of water that is produced:
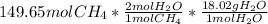
Which is equal to:
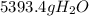
Which is not among the choices.
Regards!