Answer:
P₁ = ⅔ P₀
Step-by-step explanation:
For this exercise we can use the ideal gas equation
PV = n R T
let's write the equation for the initial point, where we will use the subscript o and the end with subscript 2
Suppose the temperature does not change in the process
P₀V₀ / n₀ = P₁V₁ / n₁
They tell us that
V₁ = V₀ / 2
n₁ = n₀ / 3
we substitute
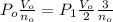
P₀ =
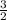 P₁
P₁
P₁ = ⅔ P₀