Answer:
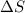 < 0
< 0
Step-by-step explanation:
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
For the reaction
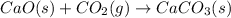
In this reaction gaseous reactants are converting to solid products. The randomness will decrease and hence entropy will also decrease. Thus
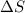 is negative or less than zero.
is negative or less than zero.