Answer:
92.6%
Step-by-step explanation:
Hello there!
In this case, according to the first-order kinetics, we can write:
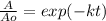
Whereas we need to know the rate constant in order to compute the percentages, given the half-life:
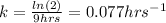
In such a way, we can now compute the fraction in which the reaction has been completed after 3 hrs:
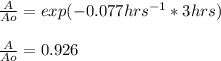
And the percent would be 92.6% after multiplying the fraction by 100 %.
Best regards!