Answer:
0.1056 mole
Step-by-step explanation:
As Sally knows that the charge on the metal ion is n = +2
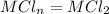
In that compartment
![$[M^(n+)]=[m^(2+)]=8.279 \ M$](https://img.qammunity.org/2022/formulas/chemistry/college/bvyafygjxznnq7rzmuxu8haryx7kv22u3m.png)
The volume of the
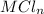 taken in that compartment = 6.380 mL
taken in that compartment = 6.380 mL
So, the number of moles of
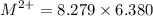
= 52.82 m mol
= 0.05280 mol
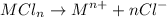
But n = 2
Therefore, moles of
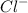 = 2 x moles of
= 2 x moles of
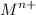
= 2 x 0.05282
= 0.1056 mole