Answer: 31.9 g of KOH can be produced from 22.2 g of KOH
Step-by-step explanation:
To calculate the moles :
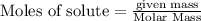
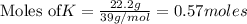
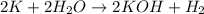
According to stoichiometry :
2 moles of
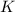 produce = 2 moles of
produce = 2 moles of
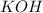
Thus 0.57 moles of
 will produce=
will produce=
 of
of
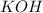
Mass of
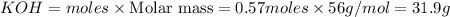
Thus 31.9 g of KOH can be produced from 22.2 g of KOH