Answer:
The temperature at 760mmHg if the volume is still 4.2 L is 400.862 K
Step-by-step explanation:
Gay Lussac's law indicates that when there is a constant volume, as the temperature increases, the pressure of the gas increases. And when the temperature is decreased, the pressure of the gas decreases. This law can be expressed mathematically as follows:
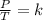
Having an initial state 1 and a final state 2, the following is true:
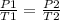
In this case:
- P1= 560 mmHg
- T1= 72 F= 295.372 K (being 32 F= 273.15 K)
- P2= 760 mmHg
- T2= ?
Replacing:
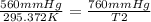
Solving:
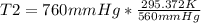
T2= 400.862 K