Answer:
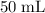 of the stock solution would be required.
of the stock solution would be required.
Step-by-step explanation:
Assume that a solution of volume
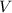 contains a solute with a concentration of
contains a solute with a concentration of
 . The quantity
. The quantity
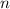 of that solute in this solution would be:
of that solute in this solution would be:
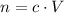 .
.
For the solution that needs to be prepared,
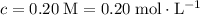 . The volume of this solution is
. The volume of this solution is
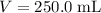 . Calculate the quantity of the solute (magnesium chloride) in the required solution:
. Calculate the quantity of the solute (magnesium chloride) in the required solution:
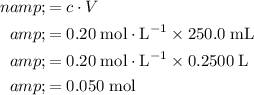 .
.
Rearrange the equation
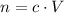 to find an expression of volume
to find an expression of volume
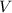 , given the concentration
, given the concentration
 and quantity
and quantity
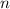 of the solute:
of the solute:
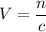 .
.
Concentration of the solute in the stock solution:
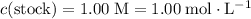 .
.
Quantity of the solute required:
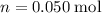 .
.
Calculate the volume of the stock solution that would contain the required
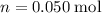 of the magnesium chloride solute:
of the magnesium chloride solute:
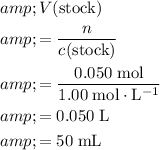 .
.