Answer: The mass of acetic acid that will be consumed is 1.512 g.
Step-by-step explanation:
Molarity is defined as the moles of solute present per liter of the solution.
The formula used to calculate the molarity of the solution is:
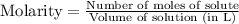
OR
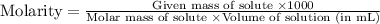 ......(1)
......(1)
Given values:
Molarity of acetic acid = 0.84 M
Volume of solution = 30 mL
Molar mass of acetic acid = 60 g/mol
Plugging values in equation 1:
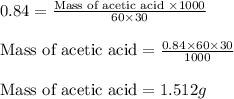
Hence, the mass of acetic acid that will be consumed is 1.512 g.