Answer:
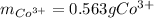
Step-by-step explanation:
Hello there!
In this case, since these mole-mass relationships are understood in terms of the moles of the atoms forming the considered compound, we first realize that the chemical formula of the cobalt (III) nitrate is Co(NO₃)₃ whereas there is a 1:1 mole ratio of the cobalt (III) ion (molar mass = 58.93 g/mol) to the entire compound. In such a way, we first compute the moles of the salt (molar mass = 58.93 g/mol) and then apply the aforementioned mole ratio to obtain the grams of the required cation:
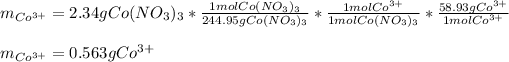
Best regards!