Answer:
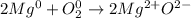
Step-by-step explanation:
Hello there!
In this case, according to the rules for the oxidation states in chemical reactions, it is possible to realize that lone elements have 0 and since magnesium is in group 2A, it forms the cation Mg⁺² as it loses electrons and oxygen is in group 6A so it forms the anion O⁻²; therefore resulting oxidation numbers are:
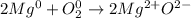
Best regards!