A chemist must prepare 0.200 L of 1.00 M aqueous silver nitrate working solution. He'll do this by pouring out 1.82 mol/L aqueous silver nitrate stock solution into a graduated cylinder and diluting it with distilled water. How many mL of the silver nitrate stock solution should the chemist pour out?
Answer: 0.110 L
Step-by-step explanation:
According to the dilution law,
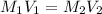
where,
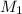 = molarity of stock silver nitrate solution = 1.82 M
= molarity of stock silver nitrate solution = 1.82 M
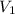 = volume of stock silver nitrate solution = ?
= volume of stock silver nitrate solution = ?
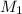 = molarity of diluted silver nitrate solution = 1.00 M
= molarity of diluted silver nitrate solution = 1.00 M
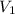 = volume of diluted silver nitrate solution = 0.200 L
= volume of diluted silver nitrate solution = 0.200 L
Putting in the values we get:
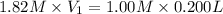
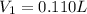
Therefore, volume of silver nitrate stock solution required is 0.110 L