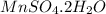Answer: The formula of the hydrate is
Step-by-step explanation:
Decomposition of hydrated manganese sulphate is given by:
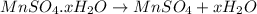
Molar mass of
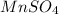 = 151.0 g/mol
= 151.0 g/mol
According to stoichiometry:
(151.0+18x) g of
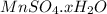 decomposes to give 151.0 g of
decomposes to give 151.0 g of
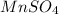
Thus 45.91 g of
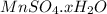 decomposes to give=
decomposes to give=
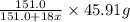 of
of
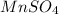
Also we are given : 37.07 g of
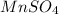 is produced
is produced
Thus we can equate the two equations:
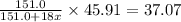
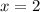
Thus the formula of the hydrate is