Answer:
Step-by-step explanation:
The standard molar mass is:
For (S )-1-chloro-4-ethyl-2-methylhexane = 162.5 g/mol
For triphenylphosphine = 262 g/mol
For ( S )-1-chloro-4-ethyl-2-methyl triphenylphosphonium = 424.5 g/mol
The mass required for 81% yield =
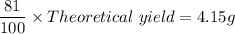
Theoretical yield =
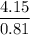
= 5.1235 g
thus, since 424.5 g yield produce from 162.5 g
∴
5.1235 g yield will produce =
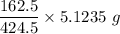
= 1.9613 g of alkyl halide (-chloro) required.
Also, since 424.5 g yield produce from 262 g phosphine
∴
5.1235 g yield will produce =
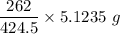
= 3.1622 g of triphenylphosphine required.