Answer:
pH = 2.462.
Step-by-step explanation:
Hello there!
In this case, according to the reaction between nitrous acid and potassium hydroxide:

It is possible to compute the moles of each reactant given their concentrations and volumes:
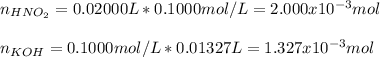
Thus, the resulting moles of nitrous acid after the reaction are:
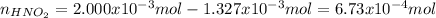
So the resulting concentration considering the final volume (20.00mL+13.27mL) is:
![[HNO_2]=(6.73x10^(-4)mol)/(0.01327L+0.02000L) =0.02023M](https://img.qammunity.org/2022/formulas/chemistry/high-school/lefmr08zbti2z1s5kx32ckkm2k7krnl22p.png)
In such a way, we can write the ionization of this weak acid to obtain:
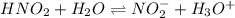
So we can set up its equilibrium expression to obtain x as the concentration of H3O+:
![Ka=([NO_2^-][H_3O^+])/([HNO_2])\\\\7.1x10^(-4)=(x^2)/(0.02023M-x)](https://img.qammunity.org/2022/formulas/chemistry/high-school/rmqycgt8utad3mewim9u8wucfs5n69ozc3.png)
Next, by solving for the two roots of x, we get:
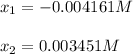
Whereas the correct value is 0.003451 M. Finally, we compute the resulting pH:
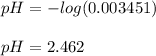
Best regards!