✧
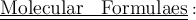
⇾
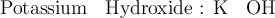
⇾
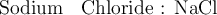
⇾
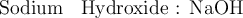
⇾
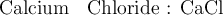
⇾
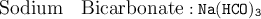
---------------------------------------------------------
☥
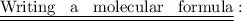
To write a molecular formula , the following steps are usually adopted only when we know the symbol and valencies of elements and radicals present in a molecule.
Step 1 : First , the name of the compound is written.
Step 2 : The symbols of basic and acidic radicals are written side by side.
Step 3 : The valency of each radical is written at the right upper corner of the symbol. The valency of one radical is transferred to another radical and it is written on the right hand side at the bottom corner. If necessary , L.C.M of the valencies us taken to get a simple whole number.
Step 4 : If a compound radical takes part in the molecular formula , the radical is enclosed in brackets and the valency number is written on the right side of the bracket at the bottom of the formula.
For instance :
 [ compound ]
[ compound ]
1.
 [ Symbol of basic and acidic radicals ]
[ Symbol of basic and acidic radicals ]
2.We know : Valencies of calcium and sulphate are 2 and 2 respectively
3.
 [ Valencies are exchanged and compound radical is enclosed in bracket ]
[ Valencies are exchanged and compound radical is enclosed in bracket ]
4.
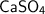 [ L.C.M is taken to get molecular formula of calcium sulphate ]
[ L.C.M is taken to get molecular formula of calcium sulphate ]
Hope I helped ! ♡
Have a wonderful day / night ! ツ
☄ Let me know if you have any questions regarding my answer !
☥
 !! ✎
!! ✎
▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁