Answer: 8855 Joules energy is released when 115 g of
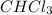 changes from a liquid to a solid at its freezing point.
changes from a liquid to a solid at its freezing point.
Step-by-step explanation:
Latent heat of freezing is the amount of heat released to convert 1 mole of liquid to solid at atmospheric pressure.
Amount of heat released to freeze 1 gram of
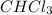 = 77 J/g
= 77 J/g
Mass of
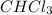 given = 115 gram
given = 115 gram
Heat released when 1 g of
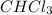 is freezed = 77 J
is freezed = 77 J
Thus Heat released when 115 g of
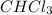 is freezed =
is freezed =
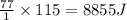
Thus 8855 Joules energy is released when 115 g of
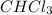 changes from a liquid to a solid at its freezing point.
changes from a liquid to a solid at its freezing point.