Answer:
Q = -33.6kcal .
Step-by-step explanation:
Hello there!
In this case, according to the equation for the calculation of the total heat of reaction when a fixed mass of a fuel like ethane is burnt, we can write:
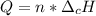
Whereas n stands for the moles and the other term for the enthalpy of combustion. Thus, for the required total heat of reaction, we first compute the moles of ethane in 3 g as shown below:
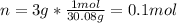
Next, we understand that -337.0kcal is the heat released by the combustion of 1 mole of ethane, therefore, to compute Q, we proceed as follows:
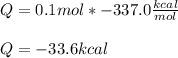
Best regards!