Answer:
m=2.0mol/kg
Step-by-step explanation:
Hello there!
In this case, according to the formula to compute molality, which requires moles of solute (HCl) and kilograms of solvent (water), we first compute the moles of the former with its molar mass as shown below:
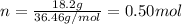
And then the kilograms of water:
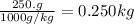
Finally, the molality turns out to be:
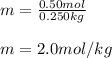
Best regards!