Answer: B. 0.36 moles
Step-by-step explanation:
According to ideal gas equation:
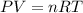
P = pressure of gas = 260 mm Hg = 0.342 atm (760 mm Hg= 1atm)
V = Volume of gas = 31.0 L
n = number of moles = ?
R = gas constant =
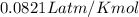
T =temperature =
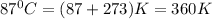
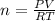
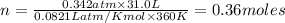
Thus the moles of gas present are 0.36