Answer:
1) The mole ratio of KClO₃ to 3O₂ is 2:3
The reciprocal of the mole ratio of KClO₃ to 3O₂ is 3:2
2) The mole ratio of K to NaNO₃ is 1:1
The reciprocal of the mole ratio of K to NaNO₃ is 1:1
Step-by-step explanation:
The mole ratio (also known as mole-mole ratio) of two compounds involved in a chemical reaction, is the ratio of the number of moles of the compounds in the reaction which can be obtained from the coefficients of the compounds in the balanced chemical reaction
The mole ratio of compound A to compound B = A:B = A/B
1)The given chemical reaction can be expressed as follows;
2KClO₃ → 2KCl + 3O₂
Therefore;
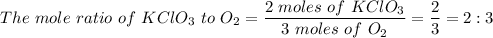
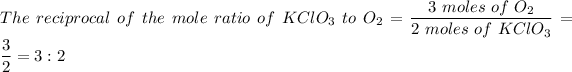
2) In the reaction NaNO₃ + K → Na + KNO₃
The mole ratio of K to NaNO₃ = 1:1
Therefore;
The reciprocal of the mole ratio of K to NaNO₃ = 1:1