Answer: The value of the equilibrium constant is 0.000023.
Step-by-step explanation:
Equilibrium concentration of
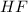 =
=
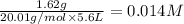
Equilibrium concentration of
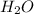 =
=
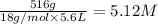
Equilibrium concentration of
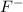 =
=
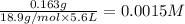
Equilibrium concentration of
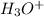 =
=
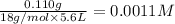
The given balanced equilibrium reaction is,
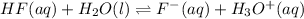
The expression for equilibrium constant for this reaction will be,
![K_c=([F^-]* [H_3O^+])/([HF]* [H_2O])](https://img.qammunity.org/2022/formulas/chemistry/college/4aaeljva10p7ngm260lbyslhvvte6zb74j.png)
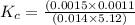
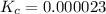
Thus the value of the equilibrium constant is 0.000023.