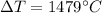Answer:
The right approach is "1479°C".
Step-by-step explanation:
The given values are:
Mass of iron piece,
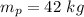
Mass of iron bucket,
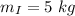
Mass of water,
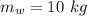
Iron's specific heat,
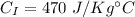
Water's specific heat,
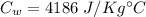
Initial temperature,
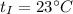
Final equilibrium temperature,
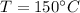
Latent heat,
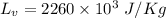
As we know,
The heat lost by the glowing piece of iron will be equal to the heat gain by the iron bucket as well as water, then
⇒
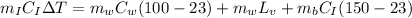
On substituting the given values, we get
⇒
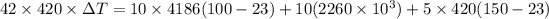
⇒
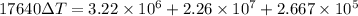
⇒
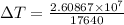
⇒