Answer:
the time it will take the element to decay to 1.9 g is 34.8 mins.
Step-by-step explanation:
Given;
half life of Nitrogen, t = 10 min
initial mass of the element, N₀ = 20 g
final mass of the element, N = 1.9 g
The time taken for the element to decay to final mass is calculated as follows;
time (min) mass remaining
0 ----------------------------------20 g
10 mins ------------------------- 10 g
20 mins ------------------------- 5 g
30 mins -------------------------- 2.5 g
40 mins --------------------------- 1.25 g
Interpolate between 2.5 g and 1.25 to obtain the time for 1.9 g
30 min ------------------------- 2.5 g
x ----------------------------------- 1.9 g
40 min -------------------------- 1.25 g
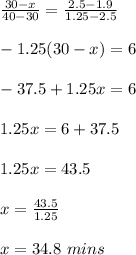
Therefore, the time it will take the element to decay to 1.9 g is 34.8 mins.