Solution :
The isentropic efficiency of the turbine is given as :
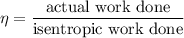
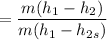
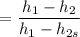
The entropy relation for the isentropic process is given by :
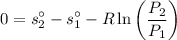
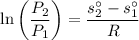
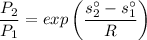

Now obtaining the properties from the ideal gas properties of air table :
At
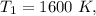
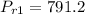
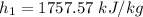
Calculating the relative pressure at state 2s :
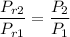
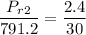
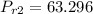
Obtaining the properties from Ideal gas properties of air table :
At
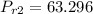 ,
,
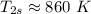
Considering the isentropic relation to calculate the actual temperature at the turbine exit, we get:
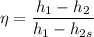
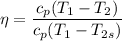
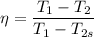
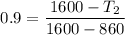
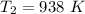
So, at
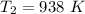 ,
,
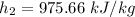
Now calculating the work developed per kg of air is :
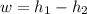
= 1757.57 - 975.66
= 781 kJ/kg
Therefore, the temperature at the exit is 938 K and work developed is 781 kJ/kg.