Answer:
[IBr] = 0.049 M.
Step-by-step explanation:
Hello there!
In this case, according to the balanced chemical reaction:
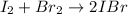
It is possible to set up the following equilibrium expression:
![K=([IBr]^2)/([I_2][Br_2]) =0.0110](https://img.qammunity.org/2022/formulas/chemistry/college/e3pj7mv3qg90t7630zgyjz9egxvl2ga7xv.png)
Whereas the the initial concentrations of both iodine and bromine are 0.50 M; and in terms of
 (reaction extent) would be:
(reaction extent) would be:
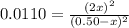
Which can be solved for
 to obtain two possible results:
to obtain two possible results:
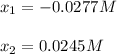
Whereas the correct result is 0.0245 M since negative results does not make any sense. Thus, the concentration of the product turns out:
![[IBr]=2x=2*0.0249M=0.049M](https://img.qammunity.org/2022/formulas/chemistry/college/88g1llj2tiegg1xg6rq52lwcobp5y1t34h.png)
Regards!