Answer:
[H⁺] = 4.56x10⁻³ M
pH = 2.34
Step-by-step explanation:
Hello there!
In this case, according to the ionization reaction of benzoic acid:
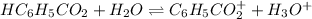
Whereas
![[H_3O^+]=[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/c9iwufidbui0ljb0pwprpcx7svj3inki0g.png) , we can set up the equilibrium expression in terms of
, we can set up the equilibrium expression in terms of
 (reaction extent) to obtain:
(reaction extent) to obtain:
![Ka=([C_6H_5CO_2^-][H_3O^+])/([HC_6H_5CO_2]) \\\\6.5x10^(-5)=(x^2)/(0.32-x)](https://img.qammunity.org/2022/formulas/chemistry/high-school/qlnm6zwm36cf9lna58c3kpb44buhxjawqx.png)
However, since Ka<<<1, we can neglect the
 on bottom to easily solve for it:
on bottom to easily solve for it:
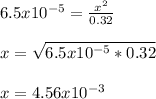
Which is actually the same as [H⁺]. Finally, the pH turns out to be:
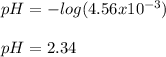
Best regards!