Answer:
The specific enthalpy of the exit stream is 63.267 Btu per pound-mass.
Step-by-step explanation:
This case represents a mixing chamber, a steady state device where two streams of the same fluid (cold and hot streams) are mixed with negigible changes in kinetic and gravitational potential energy and likewise in heat work interactions with surroundings. By Principle of Mass Conservation and First Law of Thermodynamics we have the following Mass and Energy Balances:
Mass Balance
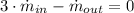 (1)
(1)
Where:
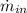 - Mass flow of the hot stream, in pounds-mass per second.
- Mass flow of the hot stream, in pounds-mass per second.
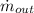 - Mass flow of the resulting stream, in pounds-mass per second.
- Mass flow of the resulting stream, in pounds-mass per second.
Energy Balance
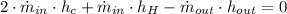 (2)
(2)
Where:
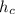 - Specific enthalpy of the cold stream, in BTU per pound-mass.
- Specific enthalpy of the cold stream, in BTU per pound-mass.
 - Specific enthalpy of the hot stream, in BTU per pound-mass.
- Specific enthalpy of the hot stream, in BTU per pound-mass.
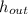 - Specific enthalpy of the resulting stream, in BTU per pound-mass.
- Specific enthalpy of the resulting stream, in BTU per pound-mass.
By applying (1) in (2) we eliminate
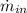 and clear
and clear
 :
:
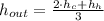
From Property Charts of the Refrigerant 134a, we have the following information:
Cold fluid (Subcooled liquid)
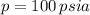
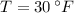
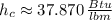
Hot fluid (Superheated steam)
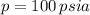
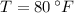
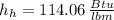
If we know that
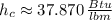 and
and
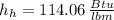 , then the specific enthalpy of the resulting stream is:
, then the specific enthalpy of the resulting stream is:
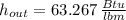
The specific enthalpy of the exit stream is 63.267 Btu per pound-mass.