Answer:
49.1 g
Step-by-step explanation:
First we convert 9.67x10²³ atoms of oxygen into moles, using Avogadro's number:
- 9.67x10²³ atoms ÷ 6.023x10²³ atoms/mol = 1.60 mol O
Then we calculate how many P₃O₉ moles there would be with 1.60 O moles:
- 1.60 mol O *
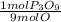 = 0.18 mol P₃O₉
= 0.18 mol P₃O₉
Finally we convert 0.18 P₃O₉ moles into grams, using its molar mass:
- 0.18 mol P₃O₉ * 237 g/mol = 49.1 g