Answer:
7.96g, 33.79%
Step-by-step explanation:
I'll try my best to explain the entire process behind this question ;)
From the question, you can write the reaction
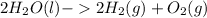
Now, there are a few reasons it is like this. Oxygen is a diatomic element, meaning it doesn't and can't exist as just O. It exists as O₂. To balance, this, double the amount of water and hydrogen so there is an equal amount of each element on both sides of the reaction (4 H's, 2 O's on the reactant side, and 4 H's, 2 O's on the product side).
From this we can get a mole-to-mole ratio.
Onto the stoichiometry. Our goal in this is to convert from grams of water to grams of hydrogen, and we do so with a mole to mole ratio.
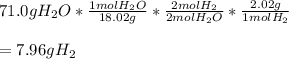
Basically, what I did was divide by water's molar mass to get moles of water, multiplied by the mole-to-mole ratio (2:2) to get moles of H2, and then multiplied by H2's molar mass to get what should be the amount of H2 produced by the reaction.
For percent yield, you can calculate it is such:
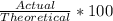
Plug the numbers in:
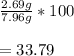
So, the percent yield is 33.79%