Answer:
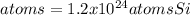
Step-by-step explanation:
Hello there!
In this case, by considering the mass-mole-particles relationships, it is possible to compute the atoms of silicon by firstly computing the moles via its atomic mass (28.1 g/mol):
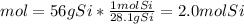
Next, via the Avogadro's number, we can compute the atoms of silicon:
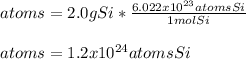
Best regards!