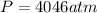Answer:
4046atm
Step-by-step explanation:
For this question you can use the ideal gas law,

Where P is pressure, V is volume, n is moles of substance, R is the constant, and T is the temperature.
Because of the units given, R will equal .08026
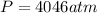
Rearrange the equation to solve for pressure:
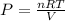
Then, plug in the values (I'll be excluding units for simplicity, but they all cancel out for pressure in atm):
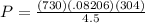
This will give you: