Answer:
387.62, 83.33%
Step-by-step explanation:
Given:
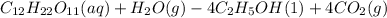
720g of Sucrose produces 323g ethanol.
Theoretical yield
To calculate the theoretical yield, you can use a mole-to-mole ratio. Assuming that there is excess H2O, you can calculate the theoretical yield like so:
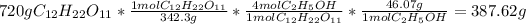
Percent Yield
An easy way to find percent yield is
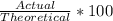
So, plug the numbers in.
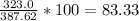
So, the percent yield is 83.33%.