Answer:
20g
Step-by-step explanation:
First of all, you have got your formula wrong. It should be
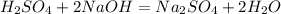 .
.
Start by finding the Relative Formula Mass for sulfuric acid.
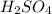 is equal to (2x1)+32+(16x4)=98. You then calculate Mols by doing
is equal to (2x1)+32+(16x4)=98. You then calculate Mols by doing
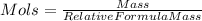 . So,
. So,
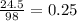 , so the Mols of Sulfuric Acid is 0.25.
, so the Mols of Sulfuric Acid is 0.25.
The Mol ratio of Sulfuric Acid to Sodium Hydroxide is 1:2, so 0.25:0.5.
Now, you have to find the Relative Formula Mass of Sodium Hydroxide, or
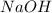 . 23+16+1=40. Finally, multiply 0.5 by 40, and you'll find that the maximum mass is 20g.
. 23+16+1=40. Finally, multiply 0.5 by 40, and you'll find that the maximum mass is 20g.