Answer:
84.3 g of nitrogen triiodide is the theoretical yield.
Step-by-step explanation:
Hello there!
In this case, according to the chemical reaction:
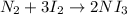
It is possible to compute the theoretical yield of nitrogen triiodide by each reactant via stoichiometry as shown below:
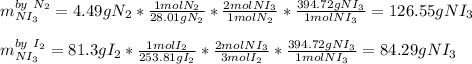
Therefore, we infer that the smallest amount is the correct theoretical yield as it comes from the limiting reactant, in this case, diatomic iodine as it yields 84.3 g (three significant figures) of nitrogen triiodide as the theoretical yield; incidentally, nitrogen acts as the excess reactant.
Best regards!