Answer:
pH = 9.475
Step-by-step explanation:
Hello there!
In this case, according to the basic ionization of the hydroxylamine:
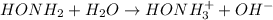
The resulting equilibrium expression would be:
![Kb=([HONH_3^+][OH^-])/([HONH_2]) =1.1x10^(-8)](https://img.qammunity.org/2022/formulas/chemistry/college/25j31ljyfjzyh8dsx9e5jebndjgh3xn3du.png)
Thus, we first need to compute the initial concentration of this base by considering its molar mass (33.03 g/mol):
![[HONH_2]_0=(1.34g/(33.03g/mol))/(0.500L) =0.0811M](https://img.qammunity.org/2022/formulas/chemistry/college/bzl2vctqhv9n6gai926s95mzr4w83gmjka.png)
Now, we introduce
 as the reaction extent which provides the concentration of the hydroxyl ions to subsequently compute the pOH:
as the reaction extent which provides the concentration of the hydroxyl ions to subsequently compute the pOH:
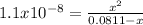
However, since Kb<<<<1, it is possible to solve for
 by easily neglecting it on the bottom to obtain:
by easily neglecting it on the bottom to obtain:
![x=[OH^-]=\sqrt{1.1x10^(-8)*0.0811}= 2.99x10^(-5)](https://img.qammunity.org/2022/formulas/chemistry/college/51zkw7mbe70y2ufku47q911vu6w3t5im3m.png)
Thus, the pOH is:
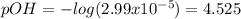
And the pH:
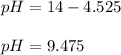
Regards!