Answer: The hydrogen-ion concentration for the aqueous solution is
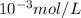 and solution is acidic.
and solution is acidic.
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxyl ion concentration.
![pOH=-\log [OH^-]](https://img.qammunity.org/2022/formulas/chemistry/high-school/eow64ghspz91qh8x39ozfikdhng1azfkyv.png)
Putting in the values:
![pOH=-\log[1* 10^(-11)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/iqntyjosd0t7toaxjl4j35qezj8k1p2j16.png)
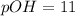
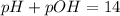
\
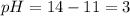
![pH=-log[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/9mqx86gdr4r9esyzqxbjxb9ugmy542eyxn.png)
![3=-log[H^+]](https://img.qammunity.org/2022/formulas/chemistry/high-school/drnch2j5tr1rug8w3nn4nqjgleuydfk3z5.png)
![[H^+]=10^(-3)M](https://img.qammunity.org/2022/formulas/chemistry/high-school/3tufl9g942h162q9g0u7e781bed1vigoj9.png)
As pH is less than 7, the solution is acidic.