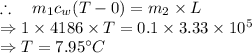Answer:
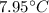
Step-by-step explanation:
Given
Mass of water is
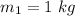
mass of ice is
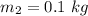
Latent heat of fusion
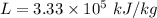
The heat capacity of water is
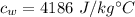
Suppose water is at
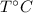 and it reaches to
and it reaches to
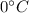 to melt the ice
to melt the ice
the heat released by water must be equivalent to heat absorbed by the ice