Answer:
Q = 282,000 J
Step-by-step explanation:
Given that,
The mass of liquid water, m = 125 g
Temperature, T = 100°C
The latent heat of vaporization, Hv = 2258 J/g.
We need to find the amount of heat needed to vaporize 125 g of liquid water. We can find it as follows :
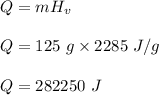
or
Q = 282,000 J
So, the required heat is 282,000 J .