Answer:
T_f = 44.6 ° C
Step-by-step explanation:
We can solve this exercise using the calorimetry relations
Q = m c_e ΔT
ΔT =
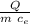
let's calculate
ΔT = -7.96 10⁴ /(0.625 4186)
ΔT = -3.04 10¹
The negative sign indicates that the temperature decreases
the temperature variation is
T_f - T₀ = ΔT
T_f = T₀ + ΔT
T_f = 75.0 - 30.4
T_f = 44.6 ° C