Answer:
The mass of oxygen is 12.10 g.
Step-by-step explanation:
The decomposition reaction of potassium chlorate is the following:
2KClO₃(s) → 2KCl(s) + 3O₂(g)
We need to find the number of moles of KClO₃:
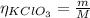
Where:
m: is the mass = 30.86 g
M: is the molar mass = 122.55 g/mol
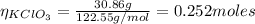
Now, we can find the number of moles of O₂ knowing that the ratio between KClO₃ and O₂ is 2:3
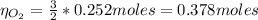
Finally, the mass of O₂ is:
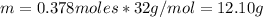
Therefore, the mass of oxygen is 12.10 g.
I hope it helps you!